
We work to raise awareness of and to find a cure for Usher 1F so that all those affected will be able to realize a future with their vision.
Our success is measured by the research we are able to fund and inspire and in the pace of that research, with treatments in development that have the potential to move from the lab to clinical trials in single digit years. These programs are fueled by our dedicated group of caring volunteers and generous donors like you.
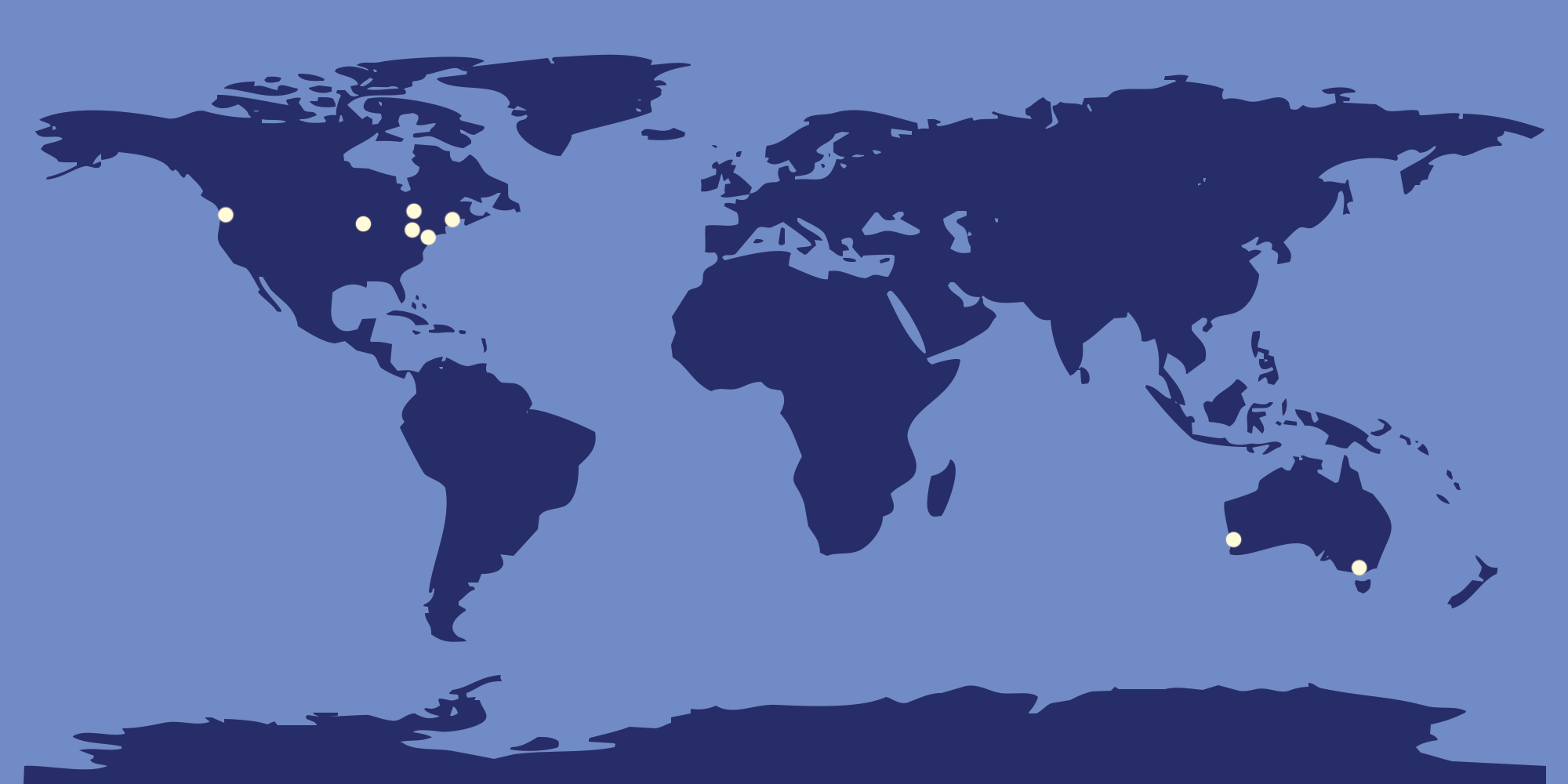
Usher 1F Research Around the World
Three Research Plans for Usher 1F
By Zollie Yavarow, PhD, MA
Usher 1F Collaborative and its research partners have secured over $14 million of funding for essential disease research across the country. With the goal of finding a treatment for Usher 1F, the foundation is taking three complimentary research approaches to support and develop treatments for Usher 1F.
Gene Therapy: Delivering the Deficient Gene
The first research approach uses the deficient protocadherin-15 (PCDH15) gene as medicine using a new technology called “gene therapy.” This approach uses a specially designed virus called AAV9 to deliver parts of the PCDH15 gene to hair cells. Dr. David Corey and his laboratory at Harvard Medical School have shown three different gene therapy approaches that lower hearing loss in a mouse model of Usher 1F. For these gene therapies to work, the hair cells must be alive so they can be rescued prior to death and complete loss. These studies use a mouse model called a conditional knockout, meaning researchers turn on the disease mutation at a chosen time. This is important because in a normal PCDH-15 knockout mouse model, the hair cells are too far gone at birth similar to humans, and the gene therapy is not able to rescue hearing. The benefit of gene therapy is that it treats the root cause of the disease, however development will take at least a couple of years and it may not help with hearing loss. Studies are underway to assess the potential for gene therapy in vision loss.
Dr. Zubair Ahmed and his laboratory at the University of Maryland Baltimore School of Medicine have also developed a gene therapy approach that is showing promise at rescuing vision in a mouse model.
Screen for Approved-drugs
The second approach looks at drugs that are already FDA-approved to treat or slow progression of Usher 1F. Drugs work through increasing or decreasing cellular functions, and changes in cellular functions are involved in many diseases. Understanding which functions are involved in Usher 1F, we can screen the approved drugs that impact these functions in hopes that one will be useful for Usher 1F. The benefit of screening approved drugs is that patients could receive them without having a clinical trial, limiting vision loss while other treatments are developed. Based on a recent study, one function to target would be health and maintenance of an energy-producing part of cells called mitochondria. Dr. Monte Westerfield and their team at University of Oregon Institute of Neuroscience have identified a compound called hexafluoro that can improve visual function and protect the retina from bright light exposure. They utilize a small fish called the Zebrafish to test this, and their model will be useful for assessing the impact of other protective compounds. Namely, they are studying antioxidants, which protect against oxidative stress that is known to be involved in retinal degeneration.
Collect Natural History Data
The third approach is collecting data on Usher 1F and its progression from patients in what is called a Natural History Study. Natural History studies are useful for understanding a disease, its progression, and the range of individual experiences. Usher 1F Collaborative has partnered with Foundation Fighting Blindness to conduct a four-year Natural History study at eleven locations around the world in a project called RUSH1F. Not only will a Natural History study increase understanding of Usher 1F, but it will also be very important for future clinical trials. Rare disease clinical trials sometimes use Natural History study data as a baseline to compare with treatment groups. This means fewer people are needed to participate in a clinical trial, which is very important for ultra rare conditions like Usher 1F. Collecting Natural History data now will make it easier to have a clinical trial to approve a new treatment. The RUSH1F Natural History study has completed enrollment with 44 participants and is in it’s third year of four.
Further reading:
Learn more about gene therapy methods for Usher 1F.
Read more about the Natural History Study and how to participate here.
Check out the clinical trial posted here.
