Since our founding in December 2013, our gifted and dedicated research scientists have worked in their labs to advance research for a cure for the vision loss of Usher 1F. We asked some of them to provide our readers with update on their progress.
Note:
PCDH15 - the gene in which mutations cause Usher 1F
R245X - the most common Usher 1F mutation
AAV (Adeno-associated virus) vector – the means of transporting genes into cells
Monte Westerfield, PhD, and Jennifer Phillips, PhD
University of Oregon Institute of Neuroscience
We are continuing to work with the USH1F fish models to understand how the loss of PCDH15 affects visual function and retinal cell health. Also, in collaboration with Dr. Jack Arbiser at Emory University, we are assessing the effect of a drug that has the potential to slow the rate of retinal cell death, using a variety of molecular tools and behavioral tests to compare treated and untreated groups of fish. Finally, we are using gene editing tools to see whether modifying the protein code to skip over the R245X mutation in PCDH15 improves the vision problems in our fish models.

Drs. Monte Westerfield and Jennifer Phillips with tanks of Usher 1F zebrafish
Photo credit: University of Oregon
David Corey, PhD
Harvard Medical School
Usher syndrome 1F is characterized by profound congenital deafness and slowly progressing vision loss, suggesting that the functional demands on PCDH15 in hair cells of the inner ear are greater than in photoreceptors of the eye. Therapeutic interventions that rescue hearing and balance in animal models are thus likely to help vision as well. Gene addition to the cochlea or retina is an attractive strategy. However, the PCDH15 coding sequence, at 5.8 kb, is too large for a single AAV vector. The Corey laboratory is developing methods for delivering a functional PCDH15 coding sequence to the inner ear in mouse models of Usher 1F, with the expectation that—if successful—these could treat vision loss as well.
In one, we split the coding sequence of mouse PCDH15 into two parts and put each part in a separate AAV vector. Once in a cell, AAV genomes can recombine to create a full-length coding sequence. To evaluate function in the inner ear in vivo, Pcdh15 conditional knockout mouse cochleas were injected with dual AAVs. Hearing tests and histological analyses were performed at P30. We found that the AAV-delivered PCDH15 was made by hair cells and transported to its normal location. While uninjected Pcdh15 conditional knockout mice were deaf and had degenerated hair bundles, mice treated with dual AAVs encoding PCDH15 demonstrated good hearing rescue at low and middle frequencies, and their bundles had a normal shape.
In another, we engineered the coding sequence of PCDH15 to remove nonessential domains, creating “mini-PCDH15s.” We shortened the extracellular part of the protein by about half but retained the essential domains at each end that bind to other proteins. The coding sequence could then fit in one AAV. Treatment of conditional knockout mice with AAVs encoding mini-PCDH15 also rescued the hearing and preserved the shape of the hair bundles.
Both strategies are promising for treatment of developing blindness in Usher 1F patients.
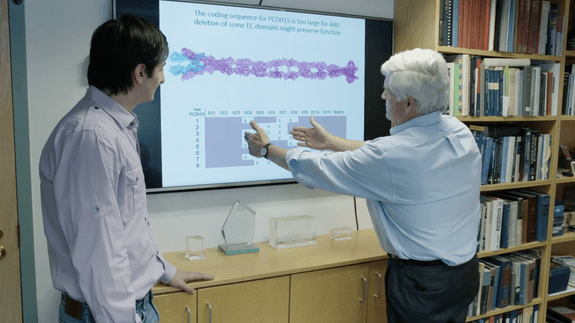
David Corey and Artur Indzhykulian studying the Usher 1F gene
Zubair Ahmed, PhD
University of Maryland School of Medicine
Our USH1F team, in collaboration with investigators at National Institutes of Health, completed a longitudinal phenotyping in thirteen USH1F individuals that revealed progressive retinal degeneration, leading to severe vision loss by the sixth decade. Around half of the studied affected individuals were legally blind by their mid-fifties. Our team also developed an USH1F mouse model and found that, similar to humans, mutant mice also had visual deficits. We are using these mice for preclinical studies for various therapeutic strategies. One of these strategies was to administer to the mice a synthetic compound that is essential for retinal functions. Intriguingly, we found much improved visual function after delivery of the synthetic compound. Taking a step forward, our research team is working on the synthesis of the compound for human consumption in order to do a clinical trial to preserve vision in USH1F patients.
In parallel, our team is also developing and investigating the impact of viral vector-based delivery of a normal copy of human PCDH15 gene in the USH1F mouse retina. Initial experiments revealed very promising results and showed significantly better visual function in the USH1F mice that have received the normal gene copy. Our team is currently exploring the long-term preservation of visual function in these animals.

Me. Sehar Riaz (graduate student) and Samuel Garmoe ( Research Assistant), Zubair Ahmed lab, are injecting viral vector constructs into mouse retina
Livia Carvalho, PhD
University of Western Australia
The Retinal Genomics and Therapy lab led by Dr Carvalho at the University of Western Australia/Lions Eye Institute, Perth, Australia, was able to secure extra funding in 2021 from the Channel 7 Telethon to continue their investigations into testing a dual AAV gene therapy for Usher 1F. Together with stem cell expert and collaborator Dr Carla Mellough, they have recruited a new student working on testing the Usher 1F gene therapy on retinal organoid cell model derived directly from an Usher 1F patient. During 2021 they were able to expand and grow Usher 1F retinal organoids in the lab and deliver the AAV gene therapy treatment. The final data analysis looking at protein and gene expression after treatment is currently underway.
Furthermore, following the already highly collaborative start of this project, which includes Prof Zubair Ahmed (University of Maryland), Assoc/Prof Luk Vandenberghe (Harvard University), Prof Alex Hewitt (University of Tasmania) and Prof Alice Pebay (University of Melbourne), Dr. Carvalho is also now working with Dr Anai Gonzalez-Cordero (Children Medical Research Institute/University of Sydney) investigating the effect of the Usher 1F dual AAV gene therapy on more mature retinal organoids carrying a different 1F mutation. This expansion will allow them to validate this therapy in Usher 1F lines from different patients, adding further confidence to the results

Carvalho lab
Vincent Tropepe, PhD
University of Toronto
The cause of retinal degeneration in USH1F is still a mystery. Using zebrafish, we have developed one of the first genetic animal models with a clear USH1F retinopathy, and this model will enable us to understand how the normal molecular function of PCDH15 leads to the production of healthy photoreceptors and typical vision. My lab has performed a detailed analysis of photoreceptor structure in animals with the mutation, and we find that the outer segments of these cells develop abnormally and often detach completely from the rest of the cell well before there is any evidence of cell loss. Funding from the USH1F Collaborative will allow us to explore two experimental approaches to molecularly repair the defective photoreceptors in our model. First, we will add back normal copies of the PCDH15 gene to see if we can rescue the defects. Second, we will use gene editing technology to directly repair the mutated gene. In both cases, we will test if repaired photoreceptors can rebuild their normal outer segments and regain visual function. If successful, this research will point to a potential window of opportunity to use PCDH15 gene therapy to repair photoreceptors at the onset of symptoms to prevent the progression of vision loss.

Vincent Tropepe
Leah Byrne, PhD
University of Pittsburgh
The Byrne Lab develops gene therapies for retinal degeneration. AAV-mediated gene replacement therapies have been proven to be effective, efficient and safe in groundbreaking clinical trials for retinal degenerative diseases such as LCA2. The packaging capacity of AAV is limited, however, to 4.7 kB, too small to package the PCDH15 gene. Our group is working to create a viral vector gene delivery system capable of efficient delivery of PCDH15. One strategy for delivering large genes involves splitting the gene into 2 halves and packaging these pieces into multiple viruses. These pieces are engineered to come back together to form a full length message after the viruses infect a cell. Unfortunately, expression from these split vector systems is less efficient than desired. We have created a system to improve split PCDH15 gene delivery, which will increase therapeutic protein expression, reduce required dosages, prevent the formation of truncated protein products, and improve the safety of gene therapy treatments. We are partnering with Dr. Zubair Ahmed to test these more efficient PCDH15 delivery systems in rodent models of Ush1F. In addition, we are developing genome editing strategies in order to directly rewrite the mutations underlying Usher1F. Our lab works in primates, in order to ensure that gene therapies developed in the lab translate into the clinic and will benefit patients.

